Review and approval
TraceX includes electronic signatures (21 CFR part 11, FDA), which are used for reviewing and approving documents.
Note: As of January 2025, authors are now required to sign documents before sending them for review or approval, with corresponding timestamps included in the document. documents that have already been reviewed cannot skip the review step when sent for approval, maintaining the proper order of signatures (Author → Reviewer → Approver). This ensures consistency and helps avoid issues during audits. The exported PDF will reflect the correct signature order based on the review sequence, ensuring proper documentation for regulatory purposes.
Reviewing a document
To send a document for review, click Send for review and select the members that will be reviewing the document. This menu will automatically populate with the reviewers selected when creating or editing the document, but you can add additional reviewers here as well. Confirm submission for review by entering your own electronic signature. Then, click Send.
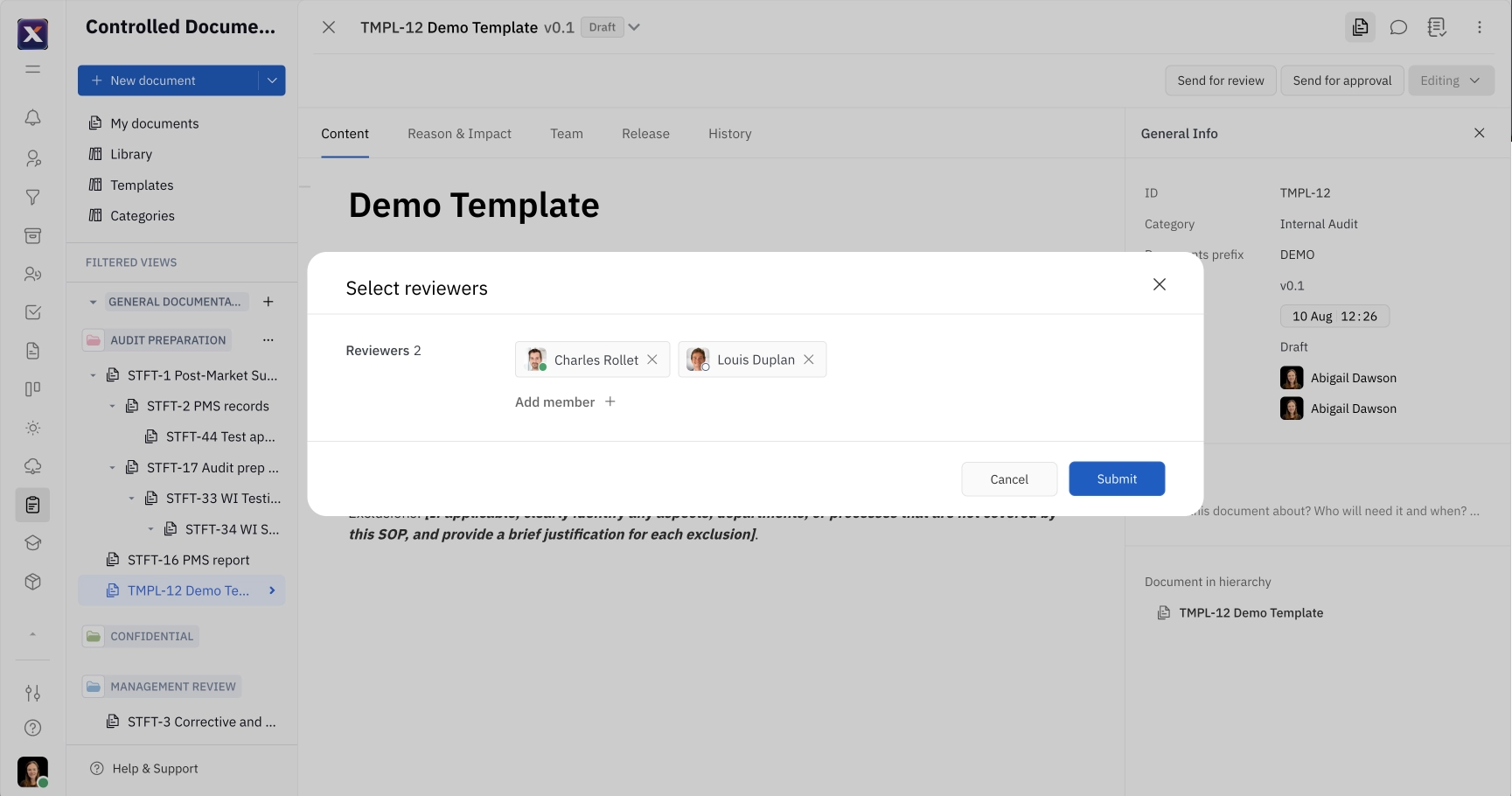
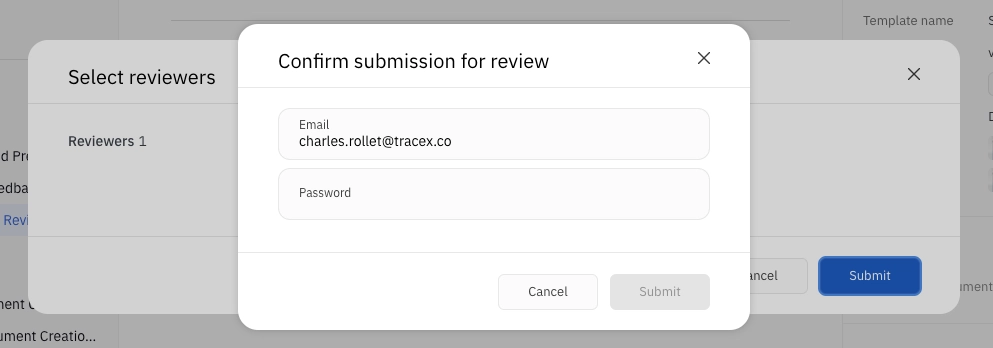
Document reviewers will be able to add their electronic signature when submitting their review.
After the document has been reviewed, any further edits will be recorded for comparison. Selecting the Comparing option will allow you to view the edits to the document, using red and green highlights to show deletions and additions.
Approving a document
To send a document for approval, click Send for approval and select the members that will approve the document. Any documents that have had changes after previously being reviewed must still be reviewed again before approval.
Note: All comments need to be resolved before sending a document for approval. If unresolved comments remain, the
Send for approvalbutton will be disabled.
Once sent, the approvers can approve or reject the document and add their electronic signature.